 Early and aggressive resection of low-grade gliomas (LGGs) leads to increased overall patient survival, decreased malignant progression, and better seizure control. This case series describes the authors’ approach to achieving optimal neurological and surgical outcomes in patients referred by outside neurosurgeons for stereotactic biopsy of tumors believed to be complex or a high surgical risk, due to their diffuse nature on neuroimaging and their obvious infiltration of functional cortex.
Early and aggressive resection of low-grade gliomas (LGGs) leads to increased overall patient survival, decreased malignant progression, and better seizure control. This case series describes the authors’ approach to achieving optimal neurological and surgical outcomes in patients referred by outside neurosurgeons for stereotactic biopsy of tumors believed to be complex or a high surgical risk, due to their diffuse nature on neuroimaging and their obvious infiltration of functional cortex.
Methods. Seven patients underwent individualized neuroimaging evaluation preoperatively, which included routine brain MRI with and without contrast administration for intraoperative neuronavigation, functional MRI with speech and motor mapping, diffusion tensor imaging to delineate white matter tracts, and MR perfusion to identify potential foci of higher grade malignancy within the tumor. Awake craniotomy with intraoperative motor and speech mapping was performed in all patients. Tumor removal was initiated through a transsylvian approach for insular lesions, and through multiple corticotomies in stimulation-confirmed noneloquent areas for all other lesions. Resection was continued until neuronavigation indicated normal brain, cortical or subcortical stimulation revealed functional cortex, or the patient began to experience a minor neurological deficit on intraoperative testing.
Results. Gross-total resection was achieved in 1 patient and subtotal resection (> 80%) in 6 patients, as assessed by postoperative MRI. Over the average follow-up duration of 31 months, no patient experienced a progression or recurrence. Long-term seizure control was excellent in 6 patients who achieved Engel Class I outcomes. Neurologically, all 7 patients experienced mild temporary deficits or seizures that completely resolved, and 1 patient continues to have mild expressive aphasia.
Conclusions. Significant resection of diffuse, infiltrating LGGs is possible, even in presumed eloquent cortex. Aggressive resection maximizes seizure control and does not necessarily cause permanent neurological deficits. Individualized preoperative neuroimaging evaluation, including tractography and awake craniotomy with intraoperative speech and motor mapping, is an essential tool in achieving these outcomes.
Low-grade gliomas account for approximately 15% of all primary brain and CNS tumors in adults.[7] These gliomas are classified based on histology and include WHO Grade I and II gliomas.[26] Grade II gliomas present their own set of therapeutic challenges and will be discussed further. The most common subtypes of Grade II gliomas are astrocytoma, oligodendroglioma, and oligoastrocytoma.[31] The majority of adults with these tumors are diagnosed between the ages of 20 and 64 years, with a median age of 39 years,[11] and the diagnosis is frequently made among otherwise healthy and productive people. Although LGGs are more histologically and radiographically benign than their high-grade counterparts, many patients eventually die of their disease due to tumor progression and/or malignant transformation.[8] The average overall survival for patients with LGGs is approximately 6 years, but up to one-fourth of patients live 20 years after diagnosis,[11] which emphasizes the importance of maintaining patient quality of life when intervening.
Favorable prognostic factors in a 2006 analysis of patients with LGGs included female sex, younger age, Caucasian race, histology, and surgery as the initial treatment.[11] Extent of resection is now a widely accepted factor that influences overall survival, progression-free survival, and malignant transformation in these gliomas.[31] Gross-total resection of LGGs may lower the rate of histological progression nearly 2-fold and alter the natural history of the disease by decreasing tumor burden and therefore its oncological potential.[8] Even if GTR is not achieved, maximal tumor resection has proven more effective than minimal resection at extending overall and progression-free survival.[36] Postoperative neurological deficits have become increasingly uncommon with the use of specialized preoperative and intraoperative neuroimaging,[30,40] as well as intraoperative motor and speech mapping.[6,12] When deficits do occur, they are usually temporary, and patients tend to recover fully without adverse affects on their long-term quality of life.[4,12,36]
Persistent seizures can also adversely affect quality of life in this patient population. Although LGGs can cause headaches or progressive neurological decline such as weakness, sensory loss, apraxia, or aphasia, 60%–80% of patients harboring LGGs initially experience a seizure; thus, seizures are the most common presenting symptom.[22,31] Generalized seizures are the most common type and are often medically refractory.[10] Some authors advocate for the use of intraoperative electrocorticography to assist in the resection of epileptogenic foci beyond the actual tumor boundaries and maximize long-term seizure control.[2,15] Given all the available data, traditional treatment options including observation with serial neuroimaging or diagnostic biopsy followed by observation, radiation, and/or chemotherapy alone, are now frequently reserved for patients with medical comorbidities who are unable to tolerate aggressive surgery.
Maintaining a satisfactory quality of life for patients with LGGs is paramount. Whenever feasible, early and maximal resection should be considered. The purpose of this case series is to describe the modalities used at our institution to achieve reasonable clinical and surgical outcomes in patients whose epileptogenic tumors were considered high surgical risk by referring providers, due to the presumed infiltration of eloquent cortex and their relative diffuse character on neuroimaging.
Study Cohort
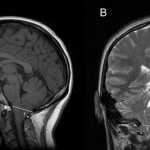
From 2008 to 2011, a total of 52 patients underwent awake cortical mapping for maximal tumor resection given the proximity of their lesions to functional cortex. Seven patients with nonenhancing or minimally enhancing mass lesions on MRI were referred for biopsy only by referring neurosurgeons due to obvious tumor invasion into the presumed functional cortex and their relative diffuse nature on imaging. These patients underwent fMRI, DTI, and MR perfusion, followed by awake craniotomy with intraoperative neurophysiological testing by the senior author (A.A.C.G.). For 6 patients this represented an initial diagnosis, and in 1 patient this was a recurrence of a previously resected LGG. All patients were primarily English speaking and did not have any significant neurological deficits, including motor, sensory, or language dysfunctions, making them eligible for demanding intraoperative evaluations. One patient suffered from mild bradykinetic hand movements as well as intermittent visual hallucinations (Case 6), and 1 demonstrated a slight expressive aphasia (Case 3).
Preoperative Neuroimaging
Patients underwent preoperative fMRI evaluation using a 3-T system (Siemens Trio Tim, Siemens Medical) with either an 8- or 12-channel array radiofrequency coil because 3-T fMRI maps more accurately correlate with intraoperative cortical stimulation than 1.5-T maps, due to increased spatial resolution.[30] Each patient was initially neurologically assessed by the radiologist, and hand dominance was determined using the Edinburgh Handedness Survey. A preprocedure practice session for each task was performed to assess patient cooperation and understanding. Field mapping was performed using axial 3.5-mm gradient echo field maps. The blood oxygen–level dependent signal, which indirectly measures neural activity via local tissue hemodynamic responses, was acquired with axial 3.5-mm single-shot gradient recalled echo–echo planar imaging during standardized task paradigms.[1,30]
Specifically, patients performed bilateral self-paced finger-tapping (gross motor test), unilateral hand/ball manipulation (fine motor test), tongue tapping, and language tasks consisting of word generation, covert naming, rhyming, and reading. Real-time prospective motion correction was performed by application of the Prospective Acquisition CorrEction (PACE) algorithm (Siemens). Moreover, during acquisition of fMRI images, continuous visual and auditory monitoring was performed as well as real-time supervision of task performance and head motion by a radiologist. The echo planar imaging fMRI raw data were processed offline using AFNI (http://afni.nimh.nih.gov/afni).[14]
The first 8 volumes were discarded to allow system equilibration, and the time series data were slice, timing, and motion corrected using standard 3D volumetric least-squares affine techniques. Time series data from the functional runs were spatially smoothed with a low-pass filter kernel of 5 mm full width at half maximum. Hemodynamic response functions for each voxel were estimated through general linear modeling; computations were performed with the use of the AFNI function 3dDeconvolve incorporating baseline correction, retrospective head motion correction using the 3D motion parameters detected in the Prospective Acquisition CorrEction algorithm, outlier correction, and detrending. The impulse response functions were computed from the deconvolution and the area under the impulse response functions converted to baseline signal change for each stimulus event. The AFNI program AlphaSim was used to compute the corrected Type I error. The criteria input to AlphaSim included voxel size (3.5 × 3.5 × 3.5 mm) and desired probability threshold (p = 0.05), and a minimum cluster size threshold of 25 voxels provided a corrected overall α of p < 0.05.[19] A Monte Carlo simulation (1000 trials) was run in AlphaSim, which outputs the corrected Type I error (α). Display thresholds for interpretation of fMRI maps were set to p < 0.001.
Each preoperative neuroimaging session also included DTI to delineate the course of critical white matter tracts[40] and DSC–MR perfusion imaging to identify tumor foci with relatively increased regional blood volume indicative of greater malignant potential.[27] Diffusion tensor imaging was performed using axial 3.5-mm spin echo–echo planar imaging acquisitions with 48 or 64 directions. Perfusion imaging was performed using a DSC technique, with online as well as calculated maps. An intravenous injection of approximately 20 ml of gadobenate dimeglumine (MultiHance, Bracco Diagnostics, Inc.) was administered for each MRI study, delivered at a rate of 4 ml/sec for perfusion purposes. Standard acquired anatomical brain MRI sequences included: precontrast sagittally acquired 1-mm 3D sampling perfection with application-optimized contrasts using different flip angle evolutions (SPACE) T2 turbo spin echo whole brain with 1-mm axial and coronal reformats; sagittally acquired 1-mm 3D T1 magnetization-prepared rapid acquisition with gradient echo (MPRAGE) with 1-mm axial and coronal reformats; axial fast low-angled shot (FLASH); sagittally acquired 1-mm 3D FLAIR whole brain with 1-mm axial reformats; axial T2 BLADE; axial 1.5-mm susceptibility weighted imaging with phase, magnitude, and thick-slab maximum intensity projection images; postcontrast sagittally acquired 1-mm 3D T1 magnetization-prepared rapid acquisition with gradient echo, with 1-mm axial and coronal reformats; and postcontrast axial FLASH. Susceptibility-weighted MR images, when available, were used to retrospectively guide correction of large, spurious hemodynamic response functions due to larger draining veins. Color-coded fMRI activation maps were acquired and overlaid onto standard 3D anatomical brain MRI sequences. All relevant anatomical MRI sequences were uploaded into our neuronavigation system and functional sequences were displayed on our largescale operating room view screens.
Awake Craniotomy and Neurophysiological Evaluation
All operations were performed in collaboration with an experienced neuroanesthesiologist and an operating team that was familiar with the workflow and goals of awake craniotomy. The patient was moved onto the operating room bed in the supine position, and intravenous access, an arterial line, and a Foley catheter (using lidocaine jelly for anesthesia) were placed while we carefully explained each step to the patient. Additionally, pneumatic compression devices were placed on the lower extremities to prevent deep venous thrombosis, and an air warmer blanket (Bair-Hugger, Augustine Medical) was placed over the patient to strictly control the temperature to 36°C. Our neuroanesthesiologist administered intravenous sedation, primarily remifentanil (0.05–0.1 μg/kg/min) and dexmedetomidine (0.7–2.0 μg/kg/hour). We infiltrated the scalp with 0.5% lidocaine with epinephrine in a 1:200,000 ratio along the trajectory of supraorbital and occipital nerves (regional scalp anesthesia) as well as at the skull clamp pin sites. The patient was then placed in a skull clamp (Mayfield, Integra LifeSciences) with 1 pin behind the ear on the side of interest and 2 pins along the contralateral superior temporal line. This method of skull clamp placement provided an ample amount of space for an ipsilateral craniotomy without any interference from the pins.
The headholder was then secured, with the head turned approximately 45° to the side opposite the surgery and the neck in a neutral position. A large exposure that includes both the lesion and the surrounding cortex is necessary for intraoperative monitoring and should be a key factor in planning the incision. A generous pterional/trauma flap incision was typically marked extending from the root of the zygoma, approximately 1 cm in front of the tragus to just beyond the midline of the forehead and behind the hairline. Neuronavigation was registered using a StealthStation TREON (Medtronic Navigation) with the surface-matching Tracer method previously described.[38] When draping, the sheets were adjusted so that the patient’s face was unobstructed and he or she had a clear view of the anesthesiologist and/or the examiner. We performed the awake craniotomy procedure as previously described by Berger and colleagues.[3]
Upon opening of the dura, the patient’s sedation was decreased, and the patient was allowed to wake up until he or she was fully conscious and cooperative. A subdural strip electrode was placed on the cortex in close proximity to the area of examination to monitor for afterdischarges. Exposed cortex underwent direct electrical stimulation with an Ojemann cortical stimulator approximately every 1 cm for approximately 2 seconds in the region of the tumor and in the immediate surrounding cortex. Stimulation intensity at each site was initiated at 2 mA and was gradually increased in 1-mA increments until a clinical response was observed, afterdischarges occurred, or a maximum of approximately 10 mA was reached.
Functional tissue was marked with plastic tags customized to that function (such as “Ha” for hand, “Le” for leg) as previously described.[39] If a seizure occurred during stimulation, cold saline was gently irrigated over the discharging cortex until the event ceased.[3] It is important not to wash away the plastic tags that have already been placed on the identified functional areas. Stimulation was then resumed at a lower threshold than that which had caused the seizure. A total of 3 noncontiguous trials were performed in each presumed eloquent area to increase the statistical probability that the stimulation finding represented real functional anatomy.[32] For motor mapping, the examiner gauged the patient’s responses by direct visualization as well as by self-reported movement by the patient upon questioning. We recommend the use of both assessments because subtle patient movement can be visually obscured by drapes and other equipment (such as intravenous lines and Bair Hugger therapy), but reported accurately by the patient. If a discrepancy occurs, fully uncovering the body part of interest and repeating stimulation can usually confirm the self-reported movement. Although no neurolinguistic standard currently exists for intraoperative testing,[13] we assessed language using a routine battery of tests for reading, naming, comprehension, and motor speech that match the fMRI paradigms and have been previously described.[32] Areas related to speech and language were highly variable between patients as previously noted.[28,32]
The operating microscope was then used to complete tumor removal based on entry corticotomies that had the least perceived morbidity on the basis of the mapping information. During the duration of tumor resection, an examiner performed brief serial neurological examinations approximately every 10 minutes on the patient. Periodically we would use direct electrical stimulation to the areas immediately adjacent to our working space to ensure that we could continue safely. Areas of language function were generously respected by leaving a 7- to 10-mm rim of cortex around the central site of importance, which has been previously recommended.[3] Subcortical mapping followed a similar procedure as cortical mapping[24] and was used to help delineate the exact location of the corticospinal tract. We continued tumor resection until normal brain was encountered as defined by neuronavigation, direct electrical stimulation revealed functional cortex, or the patient began to experience a minor deficit. We were able to resect tumor in classical areas of eloquence because these areas did not show functional activity with direct electrical stimulation,[9,17] due to the probable cortical reorganization of function in response to a slowly progressive pathological process.
For tumors with an insular extension, we completed a wide sylvian fissure dissection and removed tumor along the lateral aspect of the insula before sedation was decreased. This initial resection then determined the need for mapping of function along the inferior frontal and superior temporal gyri to find safe corridors in these regions to perform corticotomies. These corticotomies increased the working zone and improved the angles so we were able to remove tumor not accessible through the transsylvian approach.
Read full article at http://www.medscape.com/