 Three years ago, Cornelius Brown, 58, was recovering from a major brain surgery in which the muscle in her jaw was cut and a portion of her skull was removed, so a surgeon could go in and prevent an aneurysm in her brain from rupturing.
Three years ago, Cornelius Brown, 58, was recovering from a major brain surgery in which the muscle in her jaw was cut and a portion of her skull was removed, so a surgeon could go in and prevent an aneurysm in her brain from rupturing.
On Monday, Brown underwent a different procedure to prevent a second aneurysm from causing harm. But instead of an incision across her head, she has a tiny incision on her groin.
Brown was the first patient in the country to receive a new FDA-approved alternative surgical procedure.
Brown first discovered she had aneurysms when she started getting headaches in 2009.
Her primary care physician recommended she go see Dr. Adam Arthur at Semmes-Murphey where a scan of her brain revealed she had an aneurysm about the size of a pen hole on each side of her brain.
Determined too small to operate, Brown was seen every six months to a year to make sure there were no changes.
“Back in December of 2015, something shifted. I don’t know what, but they started growing,” Brown said.
The aneurysm on the right side of her brain grew to the size of a small fruit, and the first surgery was scheduled.
After Brown was put under anesthesia, Arthur, who is also the chief of neurosurgery at Methodist University Hospital, pinned her head to keep it still and cut an incision from behind her ear up to her hairline.
“She’s a beautiful woman and I didn’t want to do anything to her face,” he said.
He then cut a muscle near her temple and removed a piece of her skull, so he could go into her brain and insert a titanium clip to pinch the opening of the aneurysm. Arthur then put the skull back in and sewed her muscle and skin back together.
“I don’t want to criticize the work that was done on her because it’s my work and I’m proud of it, and she’s still a fully-functional person. She didn’t have a stroke, but for a patient,” Arthur said, “the difference between having that done, versus a little hole in her groin, you’re done, you go home the next day — if we can fix the aneurysm on the other side of her head without putting her through that, she prefers that.”
Brown was in the hospital for a week after the first surgery and in recovery for seven to eight months, undergoing both occupational and physical therapy. She had to go on short-term disability from her job as a human resources clerk at Memphis Light, Gas and Water Division, where she has worked for 13 years.
After the second procedure Monday, Brown, a born and raised Memphian who lives in South Memphis, was scheduled to go home within 72 hours.
“The time is nothing compared to three years ago,” she said.
After her first surgery, Arthur continued to monitor the aneurysm on the left side of Brown’s brain, while also working with Sequent Medical, a California-based medical device company, on a less invasive option.
Arthur had met two Sequent principals in 2011 after he gave a presentation at a medical conference in Europe.
They were Tom Wilder, president and CEO of Sequent Medical Inc., and Dr. Bill Patterson, who developed the Woven EndoBridge (WEB), an intravascular therapy for aneurysms.
They asked if he’d be the principal investigator on the clinical trial for WEB.
“No one had ever asked me to run an international trial at multiple, different centers,” Arthur said.
Arthur accepted and began what would be a more than five-year effort to perform the first FDA-approved surgery on Brown. The WEB has been available in Europe since 2010.
Methodist University Hospital was the first among the 27 clinical sites to enroll a patient, and Arthur was the first doctor to perform the surgery in August 2014. Those who enrolled in the clinical trial had a minimum year of follow up to make sure their aneurysms were fixed.
In September 2018, Arthur and Sequent Medical executives presented their findings to an FDA panel in Washington, D.C., who voted for the device’s approval.
When Brown was considering the WEB device for her second surgery, Arthur described the procedure and showed her a small woven metal basket that would fill her aneurysm and prevent it from rupturing.
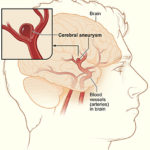
The WEB is designed to treat aneurysms that occur at bifurcations or terminuses, where the artery splits into two other arteries and an aneurysm grows off the top.
“The arteries are God’s or nature’s own way of getting things to everywhere in your body, so you hop on the freeway and get to anywhere you need to go,” Arthur said.
Using a catheter inserted through either the groin or wrist, Arthur guides the WEB, a small sphere made out of woven metal fibers, to the aneurysm and blocks the blood flow to prevent a rupture that could cause a stroke.
“The results of the trial, which we’re getting ready to publish, are really good — 150 patients, an outstanding safety record and a very good effectiveness record,” Arthur said. “It’s ironic we did the first trial at (Methodist) University Hospital and the first commercial case right here at University Hospital in Memphis.”
The risk, like with the traditional method, is the potential for the aneurysm to rupture during the procedure, or for one of the arteries to be unintentionally blocked, resulting in stroke.
“That’s the risk with anything you do. If you’re sticking things in people’s brains you can screw it up,” Arthur said. “I speak from personal experience.”
More than 40 doctors from around the country are in Memphis watching the first procedures through a live-stream at the Medical Education & Research Institute (MERI) across the street, so they can learn how to perform the procedure.
The second set of cases will be performed beginning Thursday in New York and expand to other cities thereafter. There are about 100 surgeries scheduled in the next month.
Five doctors in the Methodist Le Bonheur Healthcare system are trained and available to perform the WEB procedure.
“I put my trust in God and then Dr. Arthur,” Brown said. “When I first got here (Monday) morning, I kept thinking about three years ago for some reason. I wasn’t fearful, but I kept thinking about how he wouldn’t have to go into my skull the way he did. I’m so grateful.”