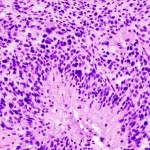
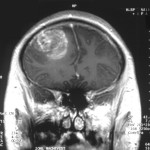
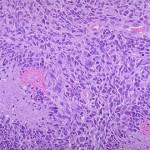
The US Food and Drug Administration (FDA) and ImmunoCellular Therapeutics have agreed on a Special Protocol Assessment (SPA) for the latter’s upcoming Phase 3 trial of its ICT-107 product candidate in newly diagnosed glioblastoma multiforme (GBM).1
An SPA is a written agreement between a drug manufacturer and the FDA regarding clinical trial design (clinical endpoints, size, statistical methodology, etc.). An SPA endorsement from the FDA constitutes, by extension, a de facto endorsement of the clinical trial protocol being reviewed. The SPA process is designed for Phase 3 trials whose data will form the primary basis for biologic licensing application (BLA) or new drug application (NDA) efficacy claims. Final marketing approval of prospective therapies, of course, remains contingent on the actual results of their respective Phase 3 investigations. 2,3
Pharmaceutical companies are not required to reach an SPA agreement with the FDA, nor are they required to comply with the FDA recommendations furnished therein. Indeed, SPA is initiated solely via company request. However, due to the investments of time and money demanded to facilitate late-stage research, manufacturers are highly incentivized to submit to SPA oversight and advice as a means of markedly mitigating the inherent risks associated with new drug development.3… [Continue Reading]