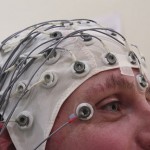
A headband delivering electrical nerve stimulation can prevent onset of migraine headaches and can be marketed for that purpose in the U.S., the FDA said Tuesday.
A headband delivering electrical nerve stimulation can prevent onset of migraine headaches and can be marketed for that purpose in the U.S., the FDA said Tuesday.
Called Cefaly, the Belgian-made device is the first to win FDA approval for migraine prevention and is also the first transcutaneous electrical nerve stimulation (TENS) system OK’d for any type of pain prevention, as opposed to acute treatment, the agency said.
The device is battery-powered and worn around the head, with the actual TENS stimulator centered on the forehead just above the eyes. It delivers a small, steady current to trigeminal nerve branches. Patients will be instructed to use the device once daily for a maximum of 20 minutes. It is approved for adults only.